The underlying
problem
Why has the international
pharmaceutical industry (‘Pharma’) been pressing so hard to get EU law changed,
to allow Direct-To-Consumer promotion of prescription-only medicines, linking it to talk
of health education and “The Expert Patient”? The short answer is that Pharma is
in crisis and has become unsustainable. Companies are no longer able to innovate enough to
grow, a problem so far largely obscured by rounds of mergers and acquisitions. It is now
clear that Pharma can survive in its present shape only by expanding markets and selling
‘blockbuster drugs’ – which in turn demands Direct-To-Consumer (DTC)
advertising and promotion.
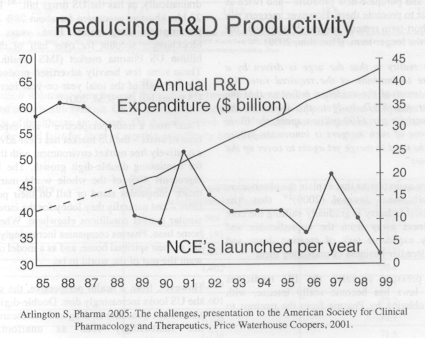
This Table illustrates a critical and
worsening situation (Price Waterhouse Coopers, 2001). The underlying problem is that the
major companies depend for their future prosperity and independence on pharmaceutical
innovation, but new drugs aren’t coming through at anything like the required rate.
Hence Pharma’s growing dependence on blockbuster drugs (products with sales of
US$500m/year or more) and the increasing investment needed to promote them. But this too
is an uphill struggle: huge investments in marketing are required, yet fewer than 4% of
all NCEs (New Chemical Entities) achieve ‘blockbuster’ status.
The situation has been getting
progressively harder for Pharma, especially in the last ten years. Even the most
successful companies are managing to develop no more than half the number of NCEs they
need to survive.
“The main issue is that, in order
to sustain average industry growth, a company has to introduce each year one new product
that will sell around £300 million per year for every 1-1.5% it has of the world
pharmaceutical market … A company the size of the newly merged GlaxoWellcome/
SmithKline Beecham needs 3 – 7 products each year, while one the size of AstraZeneca
needs 2 – 4 products each year. The problem is that research productivity is failing.
None of the major companies is close to hitting the target.” (Horrobin, 2000) [i]
“John Niblack, vice-chairman and
president of global R&D, said Pfizer could only bring two drugs to market a year
– not enough to continue to grow revenues and earnings at the rate investors have
come to expect … Mr Niblack said in an interview: ‘Two drugs a year is probably
not quite enough at this stage of market consolidation. It’s daunting, but it’s
not just Pfizer that is facing this situation’.” (Financial Times, 2001)[ii]
This in part explains the ongoing
“market consolidation”, the relentless ‘urge to merge’. The big
international companies need more and more capital to research and purchase new products -
and twice as much as that to promote them. However, mergers can only be a short-term
remedy and may[iii]
aggravate the problem in the longer-term. (Horrobin, 2000)
“The reality is that the urge is
driven by a failure to innovate at the required rate. Yet, since most of the evidence
indicates that the larger the R&D budget, the lower is the productivity per £100
million spent, the likely outcome of such mergers is innovation failure and the need to
merge yet again to cover up the disaster”.
These are the main factors that explain
the observation in the Wall Street Journal (2000)[iv], that “the
pharmaceutical industry is gradually shifting the core of its business away from the
unpredictable and increasingly expensive task of creating drugs and toward the steadier
business of marketing them”.
Thus the pressure to relax the EU
medicines advertising laws has become really intense, with concerted lobbying by Pharma
from the treetops to grassroots.
Trade versus health imperatives
The strategy for DTCA used by the Association of the British Pharmaceutical Industry (ABPI)
has been enacted throughout Europe, especially in the major drug-producing countries. The ‘Battle
Plan’, was described in a private meeting
by the Director-General of the ABPI, and reported in the trade journal, Pharmaceutical
Marketing, as follows:
"Now the
ABPI has announced that it is launching the final stages of a campaign before it tackles
the Government and the EU head on ... It is the spearhead of a carefully thought-out
campaign. The ABPI battle plan is to employ ground troops in the form of patient support
groups, sympathetic medical opinion and healthcare professionals - known as 'stakeholders'
- which will lead the debate on the informed patient issue. This will have the effect of
weakening political, ideological and professional defences … Then the ABPI will
follow through with high-level precision strikes on specific regulatory enclaves in both
Whitehall and Brussels." (Jeffries, 2000)[v]
DTC marketing has been permitted in the
USA effectively since 1997. Since then, expenditure on advertising alone has risen from
almost nothing to around US$2.5bn/year (2000). At the same time, the number of blockbuster
drugs has also risen dramatically, as has the US drugs bill. [vi]
In 1996/97, 23 blockbusters accounted for about 28% of total US prescription drug sales.
Five years later, 69 blockbusters account for over half of the $US161 billion US Pharma
market (IMS Health, 2001)[vii].
These same few heavily advertised products account for over half of the total year-on-year
increase in the US drugs bill (NIHCM, 2001)
Thus, from a trade perspective –
and especially at a time of crisis – the US market has clear advantages, as a
relatively free market environment with the potential for continuing double-digit growth.
The US market represents 40% of the whole world market, so all major companies stand or
fall on their performance there – and naturally they lobby for the introduction of
similar market conditions elsewhere. Wherever their home base, Pharma companies
increasingly regard the US as their spiritual home, and as a model of how they want the
rest of the world to be.
However, from a health perspective, the
situation in the US looks increasingly dire. Double-digit growth in the market translates
as drug and healthcare costs that are increasingly seen as unaffordable and unsustainable
even in the USA – where only half the population has good basic healthcare cover
(OECD, 2001)[viii],
and over 40 million people have no health cover at all (WHO, 2000). Health expenditure of
this order would very rapidly cripple the kind of national health care provision enjoyed
in EU countries and elsewhere. See Tables.
More than this, it is clear that health
investment produces increasingly diminishing returns. However great the benefits for some,
and in spite of great advances in standards of clinical practice, the overall health
return on investment is now low, and the absolute costs too high. The Table also explains
why, in global terms, there are good reasons for resisting the introduction of US models
of health care. Health status in many poorer countries might be transformed, with only a
tiny fraction of this spend.
Year |
US life expectancy at birth (years) |
US health spend (as % of GDP) |
US health Spend (US$/per capita) |
US pharma companies’ research
spend (US$bn) |
US spend on pharmaceuticals etc (US$bn) |
|
|
|
|
|
|
1900 |
47.3 |
|
|
|
|
1950 |
68.2 |
|
|
|
|
1960 |
69.7 |
5.1 |
141 |
|
23.4 |
1970 |
70.8 |
7.1 |
341 |
|
42.3 |
1980 |
73.7 |
8.9 |
1,052 |
2.0 |
95.7 |
1990 |
75.4 |
12.2 |
2,689 |
8.4 |
247.4 |
1995 |
75.8 |
13.7 |
3,637 |
15.2 |
323.7 |
1997 |
76.5 |
13.4 |
3,912 |
18.6 |
375.5 |
Source: US life
expectancy at birth (both sexes, all races); Data based on the National Vital Statistics
System, from Table 28, Health, United States, 2000, page 160. US health expenditure data
compiled by OECD, from Table 114, Health, United States, 2000, page 321. Expenditure on
pharmaceutical goods from OECD Health Data, 2001. R&D investment data from
Pharmaceutical Research and Manufacturers of America, Backgrounder, January 1998, accessed
September 2001 at www.phrma.org/publications/backgrounders/development/spending.phtml
The Table below illustrates something
of the conflict between trade and health imperatives, and the relative merits of the US
and other health systems. On the one hand, US market conditions support Pharma companies
and, at best, US standards of healthcare are superb. On the other hand, the US health
system represents very poor therapeutic value for money and falls far short of meeting
community health needs.
Country
|
Total per capita health expenditure
in international dollars |
Disability-adjusted life-expectancy
(overall years) |
Greece |
964 |
72.5 |
United Kingdom |
1,193 |
71.7 |
Spain |
1,211 |
72.8 |
Israel |
1,402 |
70.4 |
Finland |
1,539 |
70.5 |
Australia |
1,601 |
73.2 |
Norway |
1,708 |
71.7 |
Belgium |
1,738 |
71.6 |
Iceland |
1,757 |
70.8 |
Japan |
1,759 |
74.5 |
Italy |
1,824 |
72.7 |
Canada |
1,836 |
72.0 |
Netherlands |
1,911 |
72.0 |
Sweden |
1,943 |
73.0 |
Austria |
1,960 |
71.6 |
Luxembourg |
1,985 |
71.1 |
France |
2,125 |
73.1 |
Germany |
2,365 |
70.4 |
Switzerland |
2,644 |
72.5 |
USA |
3,724 |
70.0 |
Source:
Statistical Annex, Tables 5, 8, The World Health Report 2000, (Geneva, World Health
Organisation, May 2000), pp. 176-182. 192-195. Omitted: Malta (70.5 years - $755/head),
Andorra (72.3 - $1,216), San Marino (72.3 - $1,301), Monaco (72.4 - $1,799). These
WHO data refer to Disability Adjusted Life Expectancy (DALE). "To calculate DALE, the
years of ill-health are weighted according to severity and subtracted from the expected
overall life expectancy to give the equivalent years of healthy life". Thus
"DALE summarises the expected number of years to be lived (for babies born in 1999)
in what might be termed the equivalent of 'full health'."
Value of innovation
Against this background, both the
European Commission and the governments of some Member States have recently taken steps to
improve market conditions for companies. The objective has been to improve the
competitiveness of the EU pharmaceutical industry and to stall the threatened exodus of
Pharma companies to the USA. This has been a high-level concern even (and perhaps
especially) in the most successful drug producing and exporting nations. “This is a
truly global industry whose companies have more choice than ever before when deciding
where to place new investment”. (Blair, 2001)[ix] [x]
In November 1999, Prime Minister Blair
met to discuss the industry’s concerns with the heads of AstraZeneca, Glaxo Wellcome
and SmithKline Beecham.[xi]
The immediate output was a 70-page Task Force report, co-chaired by Lord Hunt, UK Minister
of Health responsible for drug regulation. Published in March 2001, the report emphasised
that the UK industry was, “a jewel in the industrial crown of the UK economy,”
and that a new spirit of cooperation had begun (PICTF, 2001). [xii]
The report of the Pharmaceutical
Industry Competitiveness Task Force detailed a variety of specific measures and
commitments by government that might help Pharma to prosper – including an
undertaking to fight its corner in Europe on Completion of the Single Market. This UK
initiative also led directly to the setting up of the European task force, known as
“G10”. [xiii]
“Lord Hunt … pressed these
points of principle at the round table on European pharmaceutical industry competitiveness
hosted by Enterprise Commissioner Erkki Liikanen in Brussels in December last year. These
discussions are expected to lead to the creation of a European-level Task Force on
pharmaceutical industry competitiveness”
Pharma has not of course pressed its
case for market liberalisation in Europe by pointing to any general decline in innovation.
The focus has been on the relative under-performance and lack of ‘innovation’ in
Europe, and on the threat of Pharma investing elsewhere. These issues appear to have
preoccupied both the UK and EC Task Forces. Their analyses have not recognised that
industry profitability in the US is also in decline, as measured by TSR (Total Shareholder
Returns). The average TSR for the top 20 Pharma companies “has fallen by 7% over 2
year period up to February 2001” (Price Waterhouse Coopers, 2001).
Nor has Pharma attributed the decline
in innovation to any shortcomings of its own making (including being a victim of its own
success). The industry’s focus has been exclusively on external constraints and
pressures and ‘hostile’ market conditions. Pharma blames restricted market
access, tighter and more complicated regulation, increased emphasis on drug evaluation,
price controls and lack of intellectual property protection.
The evidence suggests that both the UK
Task Force and the G10 believe that the crisis in innovation exists in Europe, not in the
USA. However, this is because they make no distinction between the relatively few new
drugs that offer some worthwhile therapeutic gain, and the majority that don’t. This
distinction is critical – and to ignore it is absurd - because most new drugs offer
only marginal advantages over existing products. Fewer than one in four new drugs brings
any important therapeutic gain. (European Parliament, 1994)[xiv] (US Food and Drug
Administration, 1992-1999)[xv] (NIHCM, August 2000),[xvi]
(Prescrire International, 2001).[xvii]
The complete failure to distinguish
between more useful and less useful innovation is underlined by the “Main
Competitiveness & Performance Indicators” proposed by in the UK Task Force report
(PICTF, 2001, Table 8.1). The three main Industry Outputs are identified as:
· Proportion of world first patents
filed for marketed new drugs divided by proportion of world R&D spend
· UK-based companies’ number of
‘global top 75’ new active substances
· % of world pharmaceutical R&D
spend
Similarly, the main analysis relied on
by the EC (Gambardella etc al, 2000)[xviii] has judged the value of
innovation solely in economic terms. The analysis takes no account of therapeutic
significance, nor the value of innovation as a response to medical need (Medicins Sans
Frontières, 2001).[xix]
Both the Commission (DG Enterprise) and the UK Task Force have regarded all marketable
innovation as worthwhile. They implicitly define the best of innovation as a blockbuster
drug.
The limitations of this perspective
– from a health point of view - are underlined by examination of the therapeutic
usefulness of the best selling US brands. Most are treatments for ‘diseases of
affluence’; relatively few represent important therapeutic gains. The difference
between economic and therapeutic breakthroughs is profound. Really effective drugs
advertise themselves – albeit not as explosively as the manufacturers would want and
need.
“Global comparisons show Europe
spends a relatively large amount of its pharmaceutical budget on older pharmaceuticals.
Thus we must consider ways and means to enhance investment in innovative new products by
the pharmaceutical industry.” (Liikanen, 2000)[xx]
The analyses relied on by the UK Task
Force and DG Enterprise not only take for granted that new licensed drugs are better
medicinal products, but also fail to ask whether the costs of innovation are or should be
as high as Pharma claims. A detailed and critical review by the Washington DC-based NGO,
Public Citizen, (2001)[xxi]
gives much evidence to suggest that the true cost of R&D is much lower than Pharma
claims.
“This new Public Citizen report
reveals how major U.S. drug companies and their Washington D.C. lobby group, the
Pharmaceutical Research and Manufacturers of America (PhRMA) have carried out a misleading
campaign to scare policy makers and the public. PhRMA’s central claim is that the
industry needs extraordinary profits to fund expensive, risky and innovative research and
development (R&D) for new drugs. If anything is done to moderate prices or profits,
R&D will suffer, and, as PhRMA’s president recently claimed, ‘it’s
going to harm millions of Americans with life-threatening conditions.’ But this
R&D scare card – or canard – is built on myths, falsehoods and
misunderstandings, all of which are made possible by the drug industry’s staunch
refusal to open its R&D records to congressional investigators or other independent
auditors.
“Using government studies,
company filings with the US Securities Exchange Commission and documents obtained by the
Freedom of Information Act”, Public Citizen’s report estimates “that the
drug industry’s claim that R&D costs total $500 million for each new drug
(including failures) is highly misleading” and that “the actual after-tax cash
outlay – or what drug companies really spend on R&D … is approximately $110
million”.
Private consultations
The watershed for DTC advertising in
the US came in August 1997, when the FDA relaxed its rules for advertising on TV, radio
and the Internet. By the end of 1997, the significance of DTCA was being discussed at
Commissioner level in Europe.[xxii] Note that this was not because of demand from
health interests within the EU. An EC evaluation of the operation of the Community
medicines control system, 1995 – 1999,[xxiii] reported general
satisfaction with Community advertising regulation at this time:
“The general view is that the
advertising provisions are working acceptably and there is no clear support for
consideration of any changes. Exceptions arise in relation to … the need to clarify
the dividing line between health education and promotion of prescription-only medicines to
the public”
By contrast, the demand for DTC
marketing from the US industry is clear from a prominent agenda item on the 1998 “Mid
Year Scorecard Report” of the Transatlantic Business Alliance. (The TABD is a
top-level forum joining companies and the EC, at Chief Executive/ Commissioner level. The
Dialogue was founded by the US and EU governments in 1995 “to forge a consensus
industry agenda for government action.”)
“Identified
Priority/Definition of Problem
The U.S. permits industry, subject to
strict regulatory controls, to provide information about prescription medicines direct to
consumers (DTC). Such communication is severely restricted in the EU. This causes an
inconsistency in the regulatory treatment of industry in the transatlantic marketplace,
deprives EU citizens of the right-to-know compared to their U.S. counterparts, and is out
of step with technological advances whereby all citizens are able to obtain information
about medicines from the Internet or other international sources but not within their own
countries”
DG
Enterprise got the message. In October 1998, the then Head of the Pharmaceuticals Unit
told the annual conference of the IFPMA (International Federation of Pharmaceutical
Manufacturers Federations) that preliminary talks on the possibility of lifting the ban on
DTC advertising might be expected soon. (Deboyser, 1998)[xxiv] The TABD “Charlotte
Communique” (November 1998) welcomed this and the industry then requested, “that
the Commission establish a working group to review DTC advertising issues”. [xxv]
The Dialogue then changed tack. The
Commission published a proposed directive (June 1999) suggesting strict limitations on
advertising medicines “to the general public”, also allowing Member
States to prohibit any advertising for medicines that were reimbursed.[xxvi]
This ruled out full-blown DTC advertising, but left open the door to promoting
prescription-only medicines to patients. The
TABD “Berlin Communique” (October 1999) reacted brusquely to what it suggested
was a frank denial of citizens’ rights - but also switched focus, to the needs and
interests of patients.
"EU
citizens should have the same access to health information as US citizens. The EU should
review existing regulations and work on solutions so that patients in the EU can benefit
from appropriate health information, carefully taking into account the American experience
in exploring deregulation of DTC advertisements. In this process, patients’ interests
should be safeguarded and consultation with doctors should be assured”
Meanwhile the Pharmaceuticals Unit
within DG Enterprise had begun to consult with its Pharmaceuticals Committee – the
representatives of Member States. This was a key input in a strongly business-oriented EC
Directorate, since Committee members represent their Departments of Health. Many have
professional qualifications; trade considerations would not be uppermost in their minds.
The Pharmaceuticals Unit and the Committee agreed a way forward in April 1999.
First, they agreed an
“Interpretive Guidance”[xxvii] which made a distinction
between drug information that either did, or did not, contain any inducement “to
promote the prescription, supply, sale or consumption of the medicinal product”.
Secondly, they agreed to set up a Working Group of selected interested parties, to
‘brainstorm’ the issue – acknowledged in their papers as “currently
subject of an intensive debate”.[xxviii] In the event, the
Working Group met only once, about a year later. It produced nothing much more than
agreement with the Unit’s recommendation to circulate a questionnaire to canvass more
views on the DTC marketing issue. Questions were subsequently posted on the DG Enterprise
website: none specifically referred to DTC marketing and, for the most part, they were
incomprehensible. (Medawar 2000)[xxix]
The results of this questionnaire-based
consultation have not been published. Nor, apparently, has much been communicated to
members of the Pharmaceutical Committee. The only clue to the outcome is a one-liner in
the UK Task Force report. “The government’s view is that the European ban on
direct to patient advertising of prescription medicines should remain and it sees no
appetite amongst other Member State governments for any change to this position”. [xxx]
Patient representation
In the Review of Pharmaceuticals
Legislation published in July 2001, DG Enterprise made proposals, in Article 88(2), to
remove the legal restriction on Direct-To-Consumer marketing of prescription drugs.
Central to the new proposals is the idea that such information should be provided by
companies only to meet the demands and needs of patients and patient organisations.
The proposal to permit companies to
respond to information requests from individual patients is uncontroversial, not least
because of the wealth of Pharma-sponsored information already available on the Internet.
It would not be feasible[xxxi]
to restrict access to such data, nor should the community need much protection when people
seek out information for themselves. The problem for the Community starts when people are
persistently confronted by more and less subtle drug promotions targeted at them.
What happens when commercially inspired
messages about diagnosis and treatment dominate the information diet? Will this encourage rational drug use and
understanding of the balance of benefit and risk? Will it promote drug treatments over
possibly better alternatives, including non-intervention and/or less effective and
cost-effective medical treatments? Will it overwhelm the supply of drug information from
independent sources, and compromise the editorial independence of the press and media, and
their coverage of health issues?[xxxii] To what extent will it
promote the medicalisation of everyday life? Will it make people feel healthier, or reduce
their confidence in their own abilities (and responsibility) to get better and stay well?
"The trouble is, we are being taken in by the
propaganda, and it is bad not only for the spirit of society; it will make any health-care
system, no matter how large and efficient, unworkable. If people are educated to believe
they are fundamentally fragile, always on the verge of mortal disease, perpetually in need
of health-care professionals at every side, always dependent on an imagined discipline of
‘preventive’ medicine, there can be no limit to the numbers of doctors’
offices, clinics, and hospitals required to meet the demand. ...We are, in real life, a
reasonably healthy people. Far from being ineptly put together, we are amazingly tough,
durable organisms, full of health, ready for most contingencies. The new danger to our
well-being, if we continue to listen to all the talk, is in becoming a nation of healthy
hypochondriacs, living gingerly, worrying ourselves half to death." (Thomas, 1980)[xxxiii]
These are some of the basic questions
that have barely been raised in the official deliberations on DTC marketing. The UK
authorities resisted public consultation, to the extent of defending their secrecy before
the Ombudsman (and losing the case).[xxxiv] DG Enterprise did
consult, but in mysterious ways.
The key to understanding the present EC
proposals, to allow some measure of DTC marketing, seems to lie in the emphasis placed on
meeting requests for information made by patient organisations:
“First, we say that there must
be a request from the side of patients or patient groups. I want to say that this change
has been done very much due to the repetitive demands of patient groups … What we
want to do is, as a test case, with respect to three specific disease groups, to make sure
that validated and patient-oriented information can be made available – when this
information is requested by patients or groups of patients (Liikanen, 2001)[xxxv]
“… the communication of
information related to certain medicinal products is authorised under strict conditions in
the interests of patients in order to respond to their legitimate needs … Member
States shall authorise the dissemination of information relating to certain medicinal
products … in order to respond to the expectations expressed by patient groups”
(DG Enterprise, 2001)[xxxvi]
But what kind of patient organisation
does DG Enterprise have in mind? Following the one-off ‘brainstorming’ meeting,
in March 2000, the Head of the Pharmaceuticals Unit in DG Enterprise wrote to Health
Action International (HAI)[xxxvii] to explain a shift in
policy (Brunet, 2000)[xxxviii].
The letter made it clear that the Pharmaceuticals Unit was no longer satisfied with the
input from the consumer bodies it had traditionally consulted, since they are “not
able to do what we have historically looked to them to do, namely the representation of
the interests of consumers as patients”.
The Head of the Pharmaceuticals Unit
wrote that “a number of representations” from patient groups had been made to
that effect – suggesting that HAI should “get in touch with Mr Elgie of Gamian
Europe who has also expressed, on behalf of a number of patient groups, an interest in
representing patients in this debate”. GAMIAN (Global Alliance of mental illness
advocacy organisations) was founded by Bristol-Myers Squibb. Rodney Elgie[xxxix]
had also been treasurer of the International Association of Patient Organisations (IAPO),
which was founded and funded by a consortium of 30-odd Pharma companies, operating as
Pharmaceutical Partners for Better Healthcare. [xl]
This and other evidence suggests that
the Pharma ‘Battle Plan’ - “to employ
ground troops in the form of patient support groups” - has been very
successful. There is clear and worrying evidence that European patient organisations are
coming to resemble their counterparts in the USA. The industry has gone to great and
sometimes improper lengths to get patient organisations on side – sweetening them to
an extent that might be expected to diminish fairness, integrity, transparency and healthy
competition.
· The
National Alliance for the Mentally Ill (NAMI) - “a grassroots organisation of
individuals with brain disorders and their family members” - received $11.72 million
from 18 Pharma companies, between 1996 and mid-1999. NAMI's leading donor was Eli Lilly,
maker of Prozac. They gave £2.87 million during that period. (Silverstein, 1999) [xli]
· “The drug companies have also
spent millions over the past decade to create seemingly independent groups that promote
their agenda. Last year, the industry trade group created Citizens for Better Medicare,
which has been waging a $50 million advertising campaign against a government-controlled
prescription drug benefit. And lobbying records show that the drug companies are major
backers of the Alliance for Better Medicare, which describes itself as ‘a
coalition of nearly 30 organizations representing seniors, patients, medical researchers
and innovators, doctors, hospitals, small businesses and others’.” (Gerth &
Stolberg, 2000)[xlii]
· “As chairman of the Danish
Migraine Association, I often stand up and tell the media and my fellow chairmen of other
patient organisations that we have to be extremely careful in order not to be in effect
advertising organs for the medical industry. When the Danish Migraine Association refused
to take industry 'assistance' to write and print the magazine, organise the lectures and
generally run the association, the industry, generously assisted by the research doctors,
literally created a new patient organisation as a substitute for the Migraine Association
in 1996. This was a bit too blatant to be generally accepted among informed patients and
opinion makers, but only because we did not accept the situation gracefully, and made the
press aware of our situation. I still hear about patient organisations that are literally
being taken over, and who do not even understand what goes on. Luckily we have a growing
awareness about the problem.” (Bulow-Olsen, 2000)[xliii]
· “A pharmaceutical company will
tomorrow break new ground by encouraging the public to demand that the NHS pay to make
available one of its drugs. The campaign, Action for Access, is funded by Biogen
and organised by a PR company on its behalf. It will urge multiple sclerosis sufferers to
demand their health authorities agree to prescribe beta-interferon on the NHS, a very
expensive drug, which can help some sufferers, but not all.” (Boseley, 1999)[xliv]
· In 1999, the
UK-based Patients Association joined forces with Pharmacia and Upjohn (P&U) in a
prototype DTC television campaign aimed at “raising awareness” about urinary
incontinence. The TV commercials mentioned no brand name, but encouraged patients to see
their doctor. Those who did stood a good chance of being prescribed the leading and most
heavily promoted brand, Detrusitol (tolterodine, P&U). However, tolterodine is
relatively ineffective. It would probably not help most users much more than a placebo,
and non-drug treatments would often be preferable. P&U had also repeatedly attracted
criticism for misleading advertising, notably in the USA. Between 1998 and 2000, P&U
received five warning letters from the US Food & Drug Administration. In the two
most recent, the company was taken to task for making headline claims about the
"selectively" of its product - suggesting that it acted on the bladder, with
less drying-up effects in the mouth. P&U evidence was based not on clinical trials,
but on studies with cats. In the UK, the company resisted regulatory pressure for over a
year. The Patients Association became aware of this too late: they had already supported
the TV campaign and accepted a large donation from P&U. In turn, they said a nice
thank-you to the company and gave it one of their ‘Platinum Awards’.[xlv]
DG Enterprise has yet to address the
problems that may arise from this. One is that Pharma companies get ‘two bites of the
cherry’ – pleading their own case and then having their views 'represented' in
consultations and regulatory matters by more or less captive patient representatives and
organisations. Another is the vulnerability of patient interests to overtures from
companies and trade associations. Many patient groups
struggle for survival and DTC advertising campaigns can empower them. Directly and
indirectly, such campaigns draw attention to the needs of special and deserving interests
and the disadvantages they face. For the groups that represent them, DTCA can bring in
more members, perhaps more column-inches and sound bites, greater exposure and prominence
and sense of achievement, and sometimes large grants from companies too.
The G10 has
acknowledged the problem exists, to the extent of proposing that “the Commission
should provide core funding for European Patient groups to enable them to participate
independently in the debate and decision-making on health matters at a European
level”. But how much would it help if funding were steered by DG Enterprise, when its primary
commitment is to entrepreneurship and market growth - and given its perspectives on the
relationship between trade and health?
The G10 Task Force (the majority)[xlvi]
seems to take it very much for granted that DTC marketing can provide patients with
information they want, need and would benefit from. People would become better informed,
disease awareness would grow and the stigma of illness would be assuaged. Moreover, trade
liberalisation would encourage companies to innovate the important new medicines that
people want now. Simple: win win.
Information
for patients
The
G10 consultation paper[xlvii]
states as ‘facts’, that “there is a need to review national regulations
which may inhibit the legitimate provision of factual information or disease education
literature by industry”, also that “the pharmaceutical industry can play a
crucial role as providers of reliable, factual and balanced information about their
medicines for patients that will support the appropriate and effective involvement of
patients and appropriate use of their products”
The
G10 paper offers no evidence to support this view, and there is much to refute it. In
theory, Pharma companies are well able provide information that complies with the twelve
standards for good quality patient information identified by G10. In practice, this does
not, and arguably cannot, happen. To expect substantial compliance with G10’s
‘good quality information’ standards seems hugely naïve.[xlviii]
Experience with DTC advertising in both the USA and New Zealand shows that companies
routinely push to the limits and frequently exceed them. During 1999, the FDA filed
violation notices for one in four products supported by DTC ads (McKinsey, 2000) [xlix]
Levels of non-compliance been reported in New Zealand have been higher still.[l]
The
complexities of enforcement are clearly great, because of the depth of scrutiny needed to
correct unbalanced representation. The pressure for ‘medicalisation’ seems
almost unstoppable, as the following example shows. It concerns a complaint made by the
FDA (16 Nov 2000) about a 60-second DTC advertisement on TV for Sarafem® (fluoxetine)
tablets. The FDA found it "lacking in fair balance and misleading":
"The graphics of the
advertisement show a frustrated woman trying to pull her shopping cart out of its
interlocked line-up in front of a store. The concurrent audio states 'Think it's PMS? It
could be PMMD." The imagery and audio presentation of the advertisement never
completely define or accurately illustrate premenstrual dysphoric disorder (PMDD) and
there is no clear distinction between premenstrual syndrome (PMS) and PMDD communicated.
Consequently, the overall message broadens the indication and trivializes the seriousness
of PMDD, a disorder whose hallmarks include a markedly depressed mood, anxiety or tension,
affective lability, and persistent anger or irritability. For a diagnosis of PMDD, these
and other symptoms must markedly interfere with work, school, usual social activities, and
relationships."
The concept of
medicalisation is hard to define. Suffice it here to recognise that the risk is real
(Thomas, 1980)[li],
and to ponder the state of Community health when runny noses present as allergic rhinitis,
headaches as migraines and forgetfulness as Alzheimer’s disease:
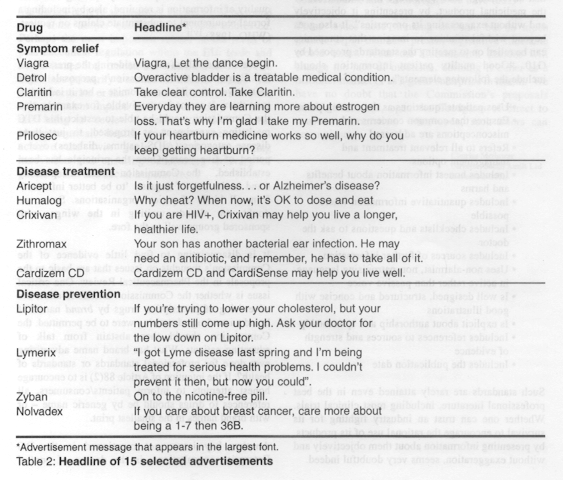
DG Enterprise
may be thinking that Europe is not about to import American-style DTCA. These headlines do
however give some indication of the level of information companies chose to provide to
prospective patients, even when strictly regulated.[lii]
It is also
worth bearing in mind that no other country has anything like the regulatory capacity of
the US Food and Drug Administration. Indeed, two-thirds of the world’s
countries that “still do not have laws to regulate pharmaceutical promotion or do not
enforce the ones they have.” (Mintzes, 1998) [liii] The implications of this for Community
Enlargement (and the Community’s commitments to Development and External Relations)
must be borne in mind. [liv]
A preliminary response
The G10 Group is now seeking to define
the distinction between “advertising” and “information to empower
patients”. It seems unlikely that distinction can ever be clear, particularly when EU
Law requires that advertising “shall encourage the rational use of the medicinal
product, by presenting it objectively and without exaggerating its properties”. It
also goes beyond wishful thinking to imagine that companies can be relied on to meeting
the standards proposed by G10. “Good quality patient information should include the
following elements”:
· Uses patients’ questions as the
starting point
· Ensures that common concerns and
misconceptions are addressed
· Refers to all relevant treatment and
management options
· Includes honest information about
benefits and harms
· Includes quantitative information where
possible
· Includes checklists and questions to
ask the doctor
· Includes sources of further information
· Uses non-alarmist, non-patronising
language in active rather than passive voice
· Is well designed, structured and
concise with good illustrations
· Is explicit about authorship and
sponsorship
· Includes references to sources and
strength of evidence
· Includes the publication date
Such standards are rarely attained
even in the best professional literature, including most clinical trials. Whether one can
trust an industry fighting for its survival to encourage the rational use of its products,
by presenting information about them objectively and without exaggeration, seems very
doubtful indeed.
The Commission could do most to empower
consumers with information, by disclosing an abundance of hard data relating to product
licensing and regulatory assessment. Indeed, there is a proposal in the Pharmaceutical
Review to release more regulatory data, but the wording makes the true intention entirely
unclear. [lv]
The revised definition of
‘advertising’, proposed in Article 86(1), seems improved by the additional
reference to information that promotes “awareness of the availability” of
products.[lvi] However, the term “advertising” itself
is unhelpfully restrictive, since it excludes a range of activities (e.g. public
relations, sponsored communications) that are basic to product marketing, and that need to
be included. What needs to be controlled is promotion – “all
informational and persuasive activities” by manufacturers and their agents “the
effect of which is to induce the prescription, supply, purchase and/or use of medicinal
drugs”. Enforcement of appropriate standards would be improved by spelling out what
quality of information is required, also by including a formal requirement to substantiate
claims on request. (WHO, 1988).[lvii]
Taken at face value (and considering
the pressure on DG Enterprise) the Commission’s proposals might seem a very sensible
compromise – but it is hard to trust them. It seems inconceivable, for example, that
the Commission would be able to restrict this DTC marketing experiment, as proposed, to
just three disease categories (AIDS, asthma, diabetes) over a period of five years. Once
the principle has been established, the
Commission could not possibly stand in the way of requests ‘to be better
informed’ from all kinds of patient organisations. Some, no doubt, are already
waiting in the wings, with sponsored groups well to the fore.
It is disappointing to find little
evidence of the Commission’s thoughts on issues that are basic to the proposals in
the Pharmaceutical Review. One critical issue is whether the Commission intends to allow
the advertising of prescription drugs by brand name?
If brand name drug advertising were to be permitted, the Commission should then
abstain from talk of educating patients. What has brand name advertising ever done for
professional standards or standards of health? If
the intention of Article 88(2) is to encourage honest attempts to inform patients/
consumers, all references to drugs should be by generic name – or with brand names in
the smallest print.
Another key issue, not mentioned in the
DG Enterprise consultations, is the degree of control envisaged over ‘permission
marketing’. Should companies be allowed to advertise to consumers who have not made
direct and specific requests to them? The rapid proliferation of DTC promotion of
medicines would be guaranteed if companies were allowed to use mass mailing lists to
advertise to anyone who had (or hadn’t) ticked a box asking for further general
information about an illness and/or possible treatments –e.g. in response to a
magazine article.
Overall, the Commission’s analysis
seems neither well-founded nor realistic. The emphasis on trade imperatives seems
excessive, as does the lack of health perspective. Most of the decision-making went on
behind closed doors, and uninhibited consultation with industry was matched by indifferent
dialogue with other interests. There are also strong grounds for suspicion that the most
heavily sponsored patient organisations have had (and will have in future) undue influence
on the decision-making process.
We do not believe satisfactory answers
will be found until some deeper questions have been addressed. Perhaps the most important
relates to the locus of pharmaceutical regulation within the EU: trade and health issues
both matter, but which comes first? Are medicines primarily a matter for trade or health
– for this Directorate or that? We are not able to share the high optimism implied in
the G10 terms – its main task being to “review the extent to which current
pharmaceutical, health and enterprise policies achieve the twin goals of both encouraging
innovation and ensuring satisfactory delivery of public health and social
imperatives.”
It seems fanciful to call them
“twin” goals – as if the success of Pharma naturally went hand-in-hand with
health. Why does the G10 consultation document invite no discussion of such issues as the
cost and quality of innovation, the blitz marketing of drugs, and the risks of
medicalisation, sponsorship and dependence? The nature of the conflict between health and
trade imperatives has not yet been properly addressed. Five members of the Task Force have
official responsibilities for Health; some distinctive contribution from them is now due. [lviii]
Does the European Community really want
to invest in innovation by investing in Big Pharma – and if so, on what terms? What
else could be done for health with US$500 million – the cost estimated by industry of
developing a single new drug? Should the Community be pouring money into future promise,
hopes and dreams - or attending more to present and future priorities and needs? How much
more could be done for health by using more effectively the understanding and technologies
we have today?
These issues need thinking through, but
in much greater depth than the present legislative timetable would allow. What the
Commission decides will change the face of medicine, defining it either as a healthier
market or as a great enterprise in health. We
have no doubt that the Commission’s proposals would, if enacted, open the floodgates
of direct to consumer promotion. Once open, such doors can never close.

